Abstract
Background
UCART19 is a genetically modified T-cell product manufactured from non-HLA matched healthy donor cells. Lentiviral-transduced CAR T-cells express (1) an anti-CD19 CAR (anti-CD19 scFv- 41BB- CD3ζ) and (2) an RQR8 "safety switch" that is intended to allow targeted elimination of RQR8+ cells by rituximab. UCART19 has been additionally modified to disrupt the T-cell receptor alpha constant (TRAC) and CD52 genes. The preliminary results of this "off-the-shelf" allogeneic CAR T-cell therapy in a phase I, dose-escalation trial of UCART19 in CD19+ R/R B-ALL adult patients (pts) are described.
Methods
The primary objective of this study is to determine the maximum tolerated dose of UCART19 by investigating up to four dose levels (DL) in separate sequential cohorts. Adult pts (age ≥16 years) with CD19+ R/R B-ALL who have exhausted available treatment options are eligible. Disease burden must be quantifiable morphologically or with a minimal residual disease (MRD) load ≥1x10-3 at the end of the last anti-leukemic treatment. The lymphodepletion regimen combines cyclophosphamide and fludarabine, with or without alemtuzumab (FC or FCA). A single dose of UCART19 is administered on Day 0, and pts are closely monitored for safety and anti-leukemic activity until the end of study, 3 months after UCART19 administration. Pts are then rolled-over into a 15-years long-term follow-up study. The dose escalation follows a modified Toxicity Probability Interval (mTPI) design based on the occurrence of dose-limiting toxicity (DLT) assessed at the end of the 28-day evaluation period post UCART19 (D28).
Results
As of 24 June 2017, the 2 first cohorts (3 pts each) who received the first DL (DL1=6x106 total CAR+ cells) have been completed. Median age was 22.5 years (range 18-42). Pts received 1 to 5 previous lines of treatment with 5 out of 6 pts having undergone an allogeneic stem cell transplant (allo-SCT). Four of them had relapsed within 4-6 months post-transplant. Prior to UCART19 infusion, 4 pts had low disease burden (<5% leukemic blasts in bone marrow (BM)) and 2 pts had high disease burden (69 and 100% blasts respectively). All pts received lymphodepletion with FCA.
All pts experienced cytokine release syndrome (CRS): 1 G1, 4 G2 and 1 G4. CRS G1 and G2 were manageable by supportive care ± tocilizumab. CRS G4, assessed as a DLT, occurred in the context of neutropenic sepsis, and was considered to be a contributory factor in the patient's death from multiple organ failure at D15. Time to onset of first CRS symptoms ranged between D5 and D10. CRS correlated with serum cytokine increase (IL-6; IL-10 and INFγ) and UCART19 expansion in the blood. One patient was reported to have probable skin GvHD G1. Only G1 neurotoxic events were observed in 1 patient. Asymptomatic viral reactivations (CMV and/or adenovirus) were seen in 3 pts and resolved with antiviral therapy.
Among the 6 pts, 4 achieved a CRi with MRD negativity at D28 (MRD-ve, defined as a tumor burden <0.01% assessed by flow cytometry and/or qPCR), 1 was refractory to treatment at D28 and 1 died at D15.
All 4 pts achieving MRD-ve remission underwent a subsequent allo-SCT, 3 of them within 3 months of UCART19 infusion and 1 following retreatment with FC lymphodepletion and the same dose of UCART19, this patient having relapsed with CD19+ disease 2 months post initial UCART19 infusion. Post allo-SCT, 1 patient relapsed at 100 days with CD19+ disease, 1 died from infection and 2 remain in complete remission.
Three pts remain alive at 2.4, 5.3 and 10.2 months respectively post UCART19 treatment.
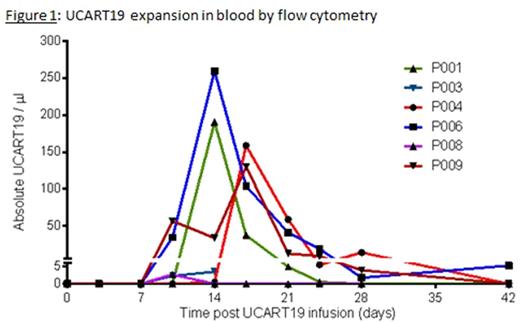
UCART19 (both cells and transgene levels) peaked between D12 and D17 in blood (flow cytometry [figure 1] and qPCR, respectively). UCART19 was detectable in blood from D10 to D28 (up to D42 in 1 patient) and in BM aspirates performed at D14 and D28. In-vivo cell expansion in BM occurred in all but the refractory patient.
Conclusion
Preliminary results of this first-in-human trial of UCART19 treatment in a high risk R/R B-ALL adult population revealed no unexpected toxicities. Asymptomatic lymphodepletion-related viral reactivations and a probable skin GvHD G1 were encountered. CRi with MRD-ve was achieved in 4 out of 5 pts who reached D28. The 2 first cohorts treated at DL1 have been completed and DL2 will now be investigated on which further results may be presented. The study is active in the UK and will be expanded to other EU countries and the US (NCT 02746952).
Graham: Servier: Research Funding; Pfizer: Other: Educational meeting attendance; Gilead: Other: Educational meeting attendance; Sanofi: Other: Educational meeting attendance. Yallop: Jazz Pharmaceuticals: Honoraria; Amgen: Honoraria; Pfizer: Other: Advisory board. Jozwik: Servier: Research Funding. Patten: Gilead Inc: Honoraria, Research Funding; Roche: Honoraria; Abbvie: Honoraria. Ellard: Moldmed: Honoraria. Potter: Pfizer: Other: Advisory board; Jazz: Honoraria. Devereux: AbbVie: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Roche: Consultancy, Other: travel expenses; GSK: Consultancy; Gilead: Consultancy, Honoraria, Other: travel expenses, Speakers Bureau; Janssen: Consultancy, Honoraria, Other: travel expenses, Speakers Bureau; Servier: Other: Advisory board. Pagliuca: Jazz: Honoraria; Merck: Honoraria, Research Funding; Bluebird: Honoraria; Pfizer: Honoraria; Basilea: Honoraria; Astellas: Consultancy, Speakers Bureau; Gilead: Honoraria. Zinai: Servier: Employment. Binlich: Servier: Employment. Dupouy: Servier: Employment. Philippe: Servier: Employment. Balandraud: Servier: Employment. Dubois: Servier: Employment. Konto: Bristol-Myers Squibb: Employment, Equity Ownership; Pfizer: Employment, Equity Ownership. Patel: Pfizer: Employment, Equity Ownership. Benjamin: Pfizer: Other: Participated in Adboard meeting, Research Funding; Servier: Research Funding; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal